Translational Neurogenetics
We are studying the molecular mechanisms of altered axon-glia cell interactions in the peripheral nerve system (PNS). We focus on the molecular understanding of Charcot-Marie-Tooth disease (CMT), a group of genetically heterogeneous rare diseases causing a broad clinical spectrum and aim to develop novel therapeutic approaches.
![]()
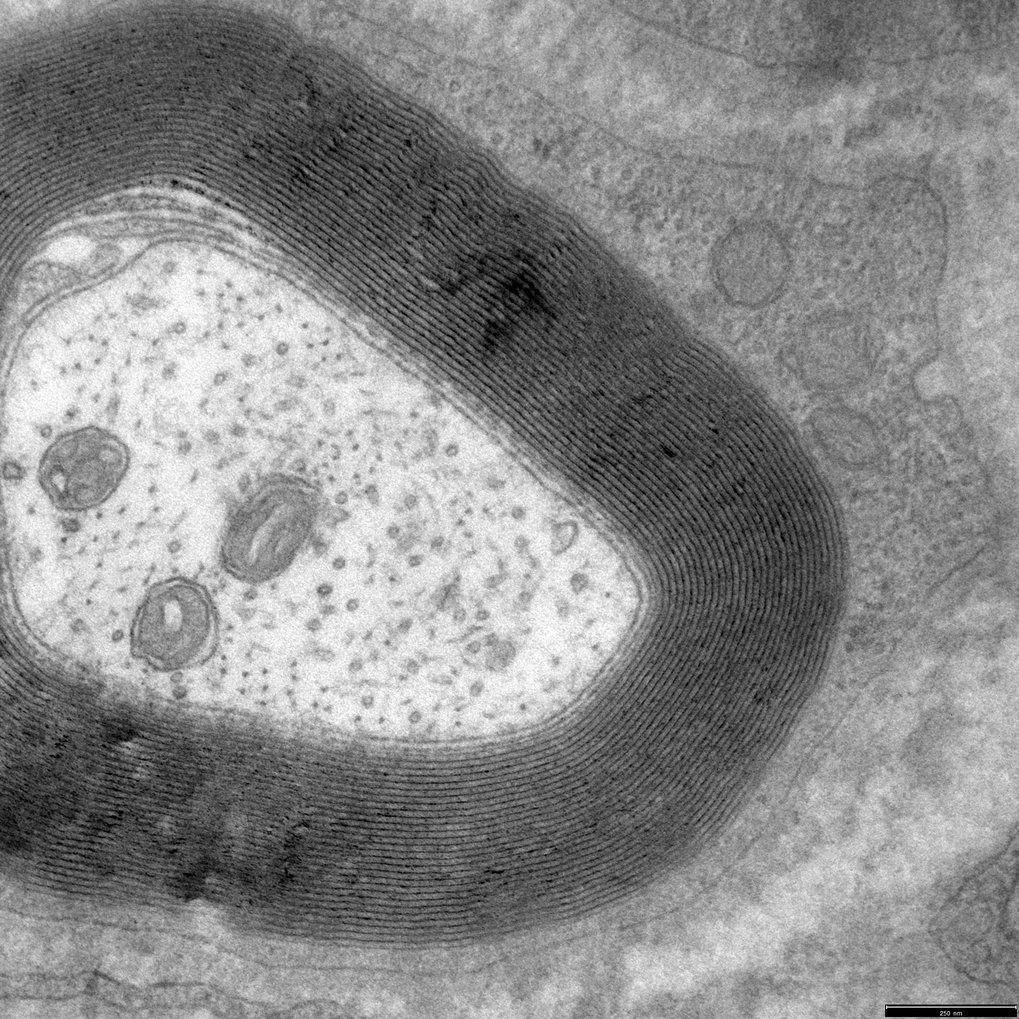 In the PNS, the myelin sheath is formed by the concentric wrapping of a Schwann cell process around the axon (HPF fixation, with Wiebke Möbius, MPI-NAT) |
![]()
![]()
Recent highlights from our basic research on PNS glial biology were: (i) that lipid supplementation in CMT1A rats is a highly effective form of therapy even preventing dysmyelination of peripheral nerves when treatment is started early postnatally (Fledrich et al., Nat Comm, 2018), a concept that can be easily translated to young patients since genetic testing of affected families has rapidly increased throughout Europe and the US. Moreover, building on the notion that (ii) Neuregulin therapy corrected the dysbalance of MAPK/PI3K signaling in CMT1A rats (Fledrich et al., Nat Med, 2014), we are now focusing on novel interaction partners of the causative protein, PMP22 -still without known function for over 30 years- that are related to signaling (Ewers et al., unpublished). Work on Neuregulin itself has been continued by (iii) identifying soluble NRG1-1 as a detrimental growth factor driving onion bulb formation in CMT, but is also likely play an important role in acquired hereditary neuropathies (Fledrich et al., Nat Comm, 2019). Another myelin related project identifies (iv) a novel role of the barely understood cytoplasmatic channels in PNS myelin (“Schmidt-Lanterman-Incisures”) for sustaining axonal function in CMT (Krauter et al., unpublished).
My dual appointment (Group leader at MPI-Nat and Consultant Neurologist at the University Medical Centre Göttingen, UMG), enables me to translate basic research projects to patients, supporting the concept of the Göttingen Campus Council (GCC). Translational projects in CMT rats examine the mechanism of a new combinatory drug, PXT-3003 (Prukop et al., 2019 and 2020) which led to phase III pivotal trial in CMT1A patients which will start Q4/2021. Unprecedented, the FDA will allow a preclinical trial in CMT rats (aimed to evaluate the effects of the individual components of PXT3003) to substitute for human trials which was highlighted in the MPG yearbook 2018. Moreover, in a novel biomarker study (building on former rat to human translational trials in skin, Fledrich et al., 2012 and 2017), we have now identified the first circulating biomarkers derived from blood in rats (at MPI of Experimental Medicine) that have been validated in over 130 CMT1A patients (at UMG) (Linhoff et al, unpublished) within the German network of CMT (CMT-NET), funded by BMBF until 2019, with over 1700 registered CMT patients as of 11/20). Clinical projects are run in parallel in my neurogenetic outpatients clinic at UMG. They include a 2-year natural history story in over 150 CMT1A patients (Krahn et al., unpublished) and a highly powered (200 participants) pain trial in CMT1A patients (Bartl et al., unpublished). The unique setting (MPI-NAT/UMG) has made Göttingen a hub for patients with hereditary neuropathies seeking novel therapeutic options from all regions in Germany.